 [Home] → [Projects] → [Swimming feather star]
[Home] → [Projects] → [Swimming feather star]
The idea for this work came to me on my first expedition to Antarctica. During a dive with our remote-controlled underwater robot, I saw these creatures, unknown to me until then, rising from the seabed and swimming away with graceful but at first glance confusing movements. Since that moment I have been fascinated by the feather stars and decided to dedicate myself to this topic in a doctoral thesis. Since this was not possible within the framework of my employment at the Alfred-Wegener-Institute, I decided to take a long and rocky road to tackle it on my own. Without the infrastructure of a university or an institute, there are not many ways to realise such a project, so this project is largely based on computer-aided possibilities, therfore I first built a small workstation for home use. I would never have expected that after five years of many hardships I finally made it and received the certificate. Nevertheless, I would like to mention that I owe this success to a whole range of people who have supported me during this time. Thank you very much for that, I really appreciate it.
A computational approach of locomotion, energy demand, and dispersal of the common comatulid crinoid Promachocrinus kerguelensis (Echinodermata) and its circum-Antarctic success, Published at the University of Oldenburg Download and in the Reports for Polar- and Marine Sciences Download
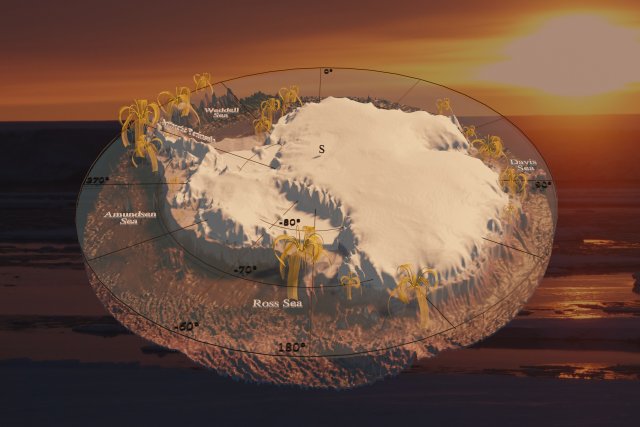
3D model of the Antarctic continent based on The International Bathymetric Chart of the Southern Ocean (IBCSO) Version 1.0,[1] displaying the so far known localities of the feather stars Promachocrinus kerguelensis. Background image showing an Antarctic sunset, taken by Nils Owsianowski.
A computational approach of locomotion, energy demand and dispersal of the common comatulid crinoid Promachocrinus kerguelensis (Echinodermata) and its circum-Antarctic success
Crinoids have existed almost unchanged for more then fout hundred million years.[2] They are found in all oceans, from the tropics[3] to polar regions,[4] and occur both in shallow coastal waters and in the deep sea.[5][6][7] Crinoids are divided into two main types: stalked crinoids (sea lilies) and their more recent family members the stalkless comatulids (feather stars). With the loss of their stalk, they acquired a free-living life accompanied by a limited ability to swim, which probably led to the marked dominance of the comatulids.[8][9][10] Among them, Promachocrinus kerguelensis is the most abundant species in the Southern Ocean, on the Antarctic continental shelf and around the sub-Antarctic islands.[4][5] Although the ecological importance of P. kerguelensis is not fully understood, they are often part of benthic communities perfectly adapted to the prevailing extreme environmental conditions.
In this work, I set up a computational approach to assess the locomotory capability of P. kerguelensis' in terms of their circum-Antarctic occurrence, their migration pattern, and the mechanisms that led to their wide dispersal. Both with regard to the advantage of their locomotory capacity and in relation to their pelagic larval drift during their ontogenetic development.
The first part of this study is devoted to a comprehensive locomotion analysis of P. kerguelensis. An idealised computational model of a swimming comatulid (Vid. 3) was derived from morphological measurements on crinoid specimens (Fig. 1) and their kinematics tracked on video footage (Vid. 1 & Vid. 2). Swimming speed and corresponding energy demand were determined with the help of analytical fluid and inverse dynamics. The most energy-efficient locomotion speed, the maximum range velocity, could be derived from their mutual dependence. The information on P. kerguelensis' swimming performance, its pelagic life phase, their cost of living, the available food as a source of energy, and the ocean and tidal currents form the basis for a migration and dispersal model in the second part of this thesis.
Video 1: ROV image of a swimming crinoid P. kerguelensis, taken in the Antarctic Weddell Sea at a depth of about 280 m.[11]
Video 2: Spline-interpolated swimming movement of the arms clarifies their paddle stroke and makes the movement pattern easier to recognise.[11]
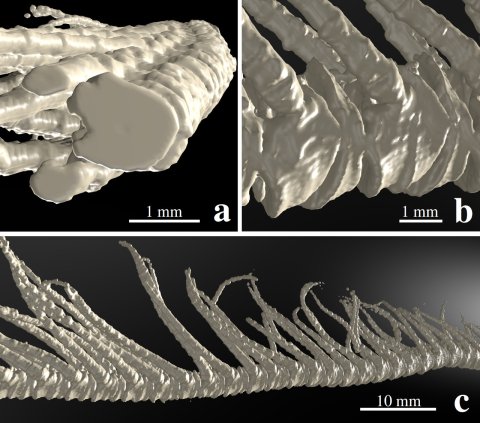
Figure 1: Computer-tomography image of an arm of the feather star Promachocrinus kerguelensis. Figure a shows the almost circular cross-section of the arm elements, b the side view of the arm segments, the so-called brachials, on which the pinnules are located laterally. These hair-like structures, about two centimetres long, can be folded out to the side or aligned to the main axis of the arm if necessary. Figure c shows an approximately six-centimetre-long central part of the arm.
Video 3: Visualisation of the underlying mathematical model of a swimming crinoid P. kerguelensis, which formed the basis for fluid mechanical calculations to determine thrust, swimming speed and energy consumption. The kinematics of the arm movement was spline-interpolated from 53 stroke cycles and arithmetically averaged; for this purpose, deviating sizes and cycle lengths of the individual paddle strokes had to be scaled both in time and dimension. Its morphological shape is based on a system of equations taken from the size ratios of 8 samples. The movement of the pinnules, the feather-like structures at the sides of the arms, was automated based on the thrust calculations. During the recovery stroke, when an arm segment is flowing against the direction of swimming, the pinnules are leaned in the direction of the arm axis to reduce the flow resistance. During the power stroke, on the other hand, which provides thrust, the pinnuls are stretched out to the side to increase the effective area as with a paddle.
Swimming performance of P. kerguelensis resulted in an energy-efficient cruising speed of ~3 cm s-1 with an energy requirement of ~12 cal h-1, which, when related to body weight, is similar to that of swimming mammals. Their daily subsistence costs are an additional ~3.5 cal day-1. Locations of potential occurrences, that at least cover their daily requirements, were derived from the net primary production. The predictions correspond very well with the locations where P. kerguelensis has been found so far (Fig. 2). Productivity analyses over a period from 2003 to 2018 show that circum-Antarctic dispersal is only possible in very productive years. Based on this constraint, the model led to circum-Antarctic settling in about 5,000 years. However, simulations leading to full dispersal required active swimming, an annual larval drift of at least 75 days and were restricted to an origin in the West Antarctic. Coastal currents promote a westerly dispersal ending in the Weddell Sea. Temporal variations of rich and lean years indicate an ongoing dynamic process sensitive to changing environmental conditions, where the circum-Antarctic dispersal of P. kerguelensis may have occurred three times since the last cold phase 15,000 years ago (Vid. 4).
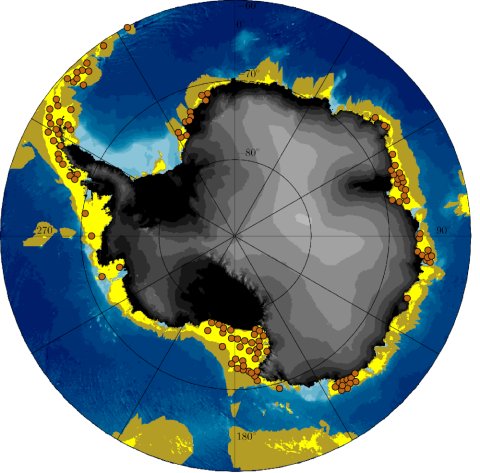
Figure 2: Stereopolar projection south 60° south based on the IBCSO chart[1]. The yellow areas represent the region that in the long term provides the calculated 3.5 calories per day that a Promachorcinus kerguelensis needs for survival and species preservation. This means that an occurrence of the feather star is at least possible due to food availability. Olive-coloured areas can theoretically be reached by means of ocean and tidal currents within 75 days of drift, in the larval stage and starting from a location within the yellow area. In contrast, the brown dots represent the actual locations where P. kerguelensis was found.
Video 4: Migration simulation originating in the Amundsen Sea southwest of the Antarctic Peninsula. The underlying model takes into account environmental parameters such as ocean and tidal currents, food supply in the form of available energy from organic carbon and nitrogen,[12] calculated from net primary production at the surface,[13][14] water depths[1] as well as species-related characteristics, energy demand for resting metabolism, reproduction and locomotion, assuming a larval phase of 75 days during which they drift with the currents in open water.[15] The simulation shows a circum-Antarctic dispersal in about 4000 years.
References
[1] Arndt, J. E. et al. (2013). The International Bathymetric Chart of the Southern Ocean (IBCSO) Version 1.0-A new bathymetric compilation covering circum-Antarctic waters. Geophysical Research Letters, 40(12):3111–3117.
[2] Guensburg, T.E., Sprinkle, J., Mooi, R. et al. Sea Lilies in Spring: Crinoid Diversification during the Early Ordovician. Paleontol. J. 55, 985–992 (2021).
[3] Ameziane, N. and Roux, M. (1997). Biodiversity and historical biogeography of stalked crinoids (Echinodermata) in the deep sea. Biodiversity and Conservation, 6(11):1557–1570.
[4] Broyer, C. D. et al. (2014). Biogeographic atlas of the Southern Ocean. The Scientific Committee on Antarctic Research, Scott Polar Research Institute, Lensfield Road, Cambridge, CB2 1ER, United Kingdom. ISBN: 978-0-948277-28-3.
[5] Clark, A. H. (1967). A Monogrphy of the existing crinoids, Vol. 1, Part 5. Number 82 in Bulletin of the United States National Museum. U. S. government printing office, Washington.
[6] Hess, H., Ausich, W., Brett, C., Simms, M. J., and Taylor, W. L. (2003). Fossil Crinoids. Cambridge University Press.
[7] Oji, T., Ogawa, Y., Hunter, A. W., and Kitazawa, K. (2009). Discovery of dense aggregations of stalked crinoids in Izu-Ogasawara Trench, Japan. Zoological Science, 26(6):406–408.
[8] Meyer, D. L. (1985). Evolutionary implications of predation on recent comatulid crinoids from the Great Barrier Reef. Paleobiology, 11(2):154–164.
[9] Meyer, D. L. and Macurda, D. B. (1977). Adaptive radiation of the comatulid crinoids. Paleobiology, 3(1):74–82.
[10] Janevski, G. A. and Baumiller, T. K. (2009). Could a stalked crinoid swim? A biomechanical model and characteristics of swimming crinoids. Society for Sedimentary Geology, 25(9):588–596.
[11] Owsianowski, Nils et al. (2017): Sea-floor videos (benthos) along ROV profile PS82/128-1 during POLARSTERN cruise PS82, links to videos. Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Bremerhaven, PANGAEA, https://doi.org/10.1594/PANGAEA.879524.
[12] Platt, T. and Irwin, B. (1973). Caloric content of phytoplankton. Limnology and Oceanography, 8(2):306–310.
[13] Morel, A. (1991). Light and marine photosynthesis: A spectral model with geochemical and climatological implications. Prog. Oceanog., 26:263–306.
[14] Antoine, D. and Morel, A. (1996). Oceanic primary production 1. Adaptation of a spectral light-photosynthesis model in view of application to satellite chlorophyll observation. Global Biogeochemical Cycles, 10(1):43–55.
[15] McClintock, J. B. and Pearse, J. S. (1987). Reproductive biology of the common antarctic crinoid Promachocrinus kerguelensis (Echinodermata: Crinoidea). Marine Biology, 96(3):375–383.
last update: 21 Oct 2023 04:44
